-
Viewpoint on 'IONS'
Viewpoint on 'Scientific Literacy'
- Proudly sponsored by
-

-
From Fascination to Reality

A deep fascination with the flight of birds inspired the invention of the airplane. A deep fascination with the stars inspired the invention of the telescope. What will fascination with metamaterials inspire?
-
Abraham vs. Minkowski 1-1

Does a photon gain or does it lose momentum when it enters a glass slab? Both may be the simple, yet ingenious answer to this centenary dilemma.
-
Thermal Images at the Nanoscale

How would you measure the temperature of a nanoscopic object? How would you build a nano-thermometer? A new technique offers a solution.
Volume 2 Story 1 - 4/8/2008
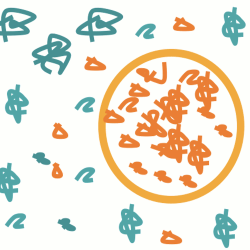
Maybe unknowingly, we both — you reading this article and I writing it — have used several polymer blends today. Car tires, foam cups, foodstuff and pharmaceutical products, all contain polymer blends. Indeed, the polymer industry is a multi-billion-euro business. Polymer blends are produced by combining two polymers. For example, there can be droplets of a certain polymer embedded in a matrix of another polymer. However, what happens if the polymers in the droplets start to escape? Unexpected runaway polymers could alter the properties of the blend and, worse than that, they can raise safety issues where edibles or medicines are concerned. In other words, you would not like to have out-of-control polymers in your coffee!
Fortunately, this does not happen – on a short time-scale and at a short distance. However, what is this time-scale? and what this distance? Now the groups led by Antonio Sasso and Stefano Guido at the University of Naples (Italy) have developed a new technique that allows us to track down the polymers escaping from a single droplet immersed into a matrix made of a different polymer.
Polymers are large worm-like molecules, composed by joining together several smaller molecules called monomers. Unfortunately, not all the desirable properties can be achieved using simple polymers. That is why, nearly as soon as chemists discovered polymers, they started experimenting with polymer blends in which two or more polymers are mixed together to create a material with intermediate properties.
A blend you may be familiar with is that which is used in plastic bottles for carbonated beverages. Two polymers, commercially known as PET and PVA, are mixed. You can easily find such acronyms on plastic bottles. PET makes the bottle strong, but it is permeable to carbon dioxide. The layers of PVA prevent carbon dioxide from getting lost and your drink from going flat. However, polymer blends also have more extraordinary applications, such as the immobilization of enzymes in the pharmaceutical industry, reverse osmosis membranes in water desalinization, and medical applications using polyelectrolyte complexes.
Even though in all these applications the assumption is that the two polymers in the blend do not mix, it is known that some chains migrate from one polymer to the other, producing an effective mixing. This effect is very slow and localized: it can occur over several days and at micrometric distances. Therefore, it is difficult, but still extremely important, to detect it.
Now, the two groups at the University of Naples have been able to see molecules escaping from a polymer called polyisobutylene (PB), embedded in a matrix of a different polymer called poly(dimetihylsiloxane) (PDMS). In an experiment, which lasted over two days, the molecules of PB were tracked as they migrated into the matrix of PDMS. Furthermore, it was also possible to measure what was happening as a function of time and distance from the boundary between the two polymers. "We tackled the problem of diffusion in polymer blends by using Raman microscopy," explains Anna Chiara De Luca from Naples University. "This technique is a well-established and powerful tool for the chemical characterization of materials. Indeed, each molecule has a very specific Raman fingerprint that allows us to distinguish it. The main advantage is that we can do this on a microscopic scale and on a single polymer droplet, whereas previous techniques were bulk techniques."
"Diffusion in polymer blends is a fascinating interdisciplinary topic," explains Stefano Guido, "and it has been tackled by several techniques, including attenuated total internal reflectance infrared spectroscopy (ATR-FTIR), nuclear magnetic resonance (NMR) imaging, and electron spin resonance (ESR) spectroscopy. These are powerful experimental techniques, but of limited spatial resolution." Therefore, it was possible to infer that polymers were migrating, but it was not possible to see their migration. "One of the main limitations of the previous techniques was the lack of spatial resolution," Guido adds, "which was making it more difficult to probe what happens near the phase boundary. In our approach we look at the actual geometry — micro-droplets dispersed in a continuous phase — that is observed in most polymer blends, and we monitor the diffusion process in situ."
"Information on the diffusion length- and time-scales from single polymer-droplets are of general interest," comments Bernhard Wolf, from the Institute of Physical Chemistry at the University of Mainz (Germany), "in at least two areas: the processing and properties of two-phase polymer blends and the fractionation of polymers involving the transport of polymer chains, which differ in their length, from a source phase into a receiving phase." "Diffusion in polymer systems is relevant in a number of applications," confirms Guido, "such as drugs delivery, the stability of medical implants, molecular transport kinetics in chemical separations, and the response of polymer-based sensor materials. Foodstuff is mostly made of biopolymers, therefore diffusion may play a very important role in safety issues such as the study of contamination kinetics by pollutants. The materials we selected can be seen as a model system, but, in principle, the techniques can be used to study more complex materials as well."

Download High Quality Video
(Quicktime 332KB)
Download High Quality Video
(Quicktime 360KB)
The Runaway Polymer
Polymer blends are allies in our everyday lives, but they can also become our worst enemies, if the polymers mix too much. It is now possible to track down the polymers' behavior at the micrometer scale.
Schematic of a polymer blend. Microdroplets of a polymer are embedded in a matrix of a different polymer. Sometimes they escape.
Fortunately, this does not happen – on a short time-scale and at a short distance. However, what is this time-scale? and what this distance? Now the groups led by Antonio Sasso and Stefano Guido at the University of Naples (Italy) have developed a new technique that allows us to track down the polymers escaping from a single droplet immersed into a matrix made of a different polymer.
Polymers are large worm-like molecules, composed by joining together several smaller molecules called monomers. Unfortunately, not all the desirable properties can be achieved using simple polymers. That is why, nearly as soon as chemists discovered polymers, they started experimenting with polymer blends in which two or more polymers are mixed together to create a material with intermediate properties.
A blend you may be familiar with is that which is used in plastic bottles for carbonated beverages. Two polymers, commercially known as PET and PVA, are mixed. You can easily find such acronyms on plastic bottles. PET makes the bottle strong, but it is permeable to carbon dioxide. The layers of PVA prevent carbon dioxide from getting lost and your drink from going flat. However, polymer blends also have more extraordinary applications, such as the immobilization of enzymes in the pharmaceutical industry, reverse osmosis membranes in water desalinization, and medical applications using polyelectrolyte complexes.
Even though in all these applications the assumption is that the two polymers in the blend do not mix, it is known that some chains migrate from one polymer to the other, producing an effective mixing. This effect is very slow and localized: it can occur over several days and at micrometric distances. Therefore, it is difficult, but still extremely important, to detect it.
Now, the two groups at the University of Naples have been able to see molecules escaping from a polymer called polyisobutylene (PB), embedded in a matrix of a different polymer called poly(dimetihylsiloxane) (PDMS). In an experiment, which lasted over two days, the molecules of PB were tracked as they migrated into the matrix of PDMS. Furthermore, it was also possible to measure what was happening as a function of time and distance from the boundary between the two polymers. "We tackled the problem of diffusion in polymer blends by using Raman microscopy," explains Anna Chiara De Luca from Naples University. "This technique is a well-established and powerful tool for the chemical characterization of materials. Indeed, each molecule has a very specific Raman fingerprint that allows us to distinguish it. The main advantage is that we can do this on a microscopic scale and on a single polymer droplet, whereas previous techniques were bulk techniques."
"Diffusion in polymer blends is a fascinating interdisciplinary topic," explains Stefano Guido, "and it has been tackled by several techniques, including attenuated total internal reflectance infrared spectroscopy (ATR-FTIR), nuclear magnetic resonance (NMR) imaging, and electron spin resonance (ESR) spectroscopy. These are powerful experimental techniques, but of limited spatial resolution." Therefore, it was possible to infer that polymers were migrating, but it was not possible to see their migration. "One of the main limitations of the previous techniques was the lack of spatial resolution," Guido adds, "which was making it more difficult to probe what happens near the phase boundary. In our approach we look at the actual geometry — micro-droplets dispersed in a continuous phase — that is observed in most polymer blends, and we monitor the diffusion process in situ."
"Information on the diffusion length- and time-scales from single polymer-droplets are of general interest," comments Bernhard Wolf, from the Institute of Physical Chemistry at the University of Mainz (Germany), "in at least two areas: the processing and properties of two-phase polymer blends and the fractionation of polymers involving the transport of polymer chains, which differ in their length, from a source phase into a receiving phase." "Diffusion in polymer systems is relevant in a number of applications," confirms Guido, "such as drugs delivery, the stability of medical implants, molecular transport kinetics in chemical separations, and the response of polymer-based sensor materials. Foodstuff is mostly made of biopolymers, therefore diffusion may play a very important role in safety issues such as the study of contamination kinetics by pollutants. The materials we selected can be seen as a model system, but, in principle, the techniques can be used to study more complex materials as well."
Giovanni Volpe
2008 © Optics & Photonics Focus
GV is currently working on his doctoral thesis at ICFO - The Institute of Photonic Sciences, Barcelona (Spain).

A. C. De Luca, G. Rusciano, G.Pesce, S. Caserta, S. Guido, and A. Sasso, Diffusion in Polymer Blends by Raman Microscopy, Macromolecules (2008) , (link).
Video 1: Polymer concentration as a function of the distance from the droplet. Acquiring Raman spectra at different distances from the droplet surface, it is possible to monitor how far the runaway polymers can reach.
Download High Quality Video
(Quicktime 332KB)
Video 2: Polymer concentration as a function of time. The smaller polymer chains escape form the droplet earlier. This is why the diffusion coefficient at a given distance from the droplet surface ia a decreasing function of time.
Download High Quality Video
(Quicktime 360KB)