-
Viewpoint on 'IONS'
Viewpoint on 'Scientific Literacy'
- Proudly sponsored by
-

-
The Photon Bouncer

Controlling quantum systems, however, is extremely subtle and difficult. The most common model currently in use, as it now turns out, is simply insufficient — a slightly more complex approach is required.
-
Happy Birthday Laser!

This year the world celebrates the 50th birthday of the laser through an initiative jointly organized by various optics and photonics organizations: the LaserFest. Happy birthday laser!
-
Nanoscopy: Shedding Light on Life

Where traditional optical microscopy fails, a new tool, the nanoscope, overcomes the last barrier: the diffraction limit. It can explore the interior of cells in 3D, non-invasively, and with nanometric resolution.
Snapshots of Electrons in Motion
Recent, groundbreaking experiments using ultrashort laser pulses have permitted the study of the motion of electrons in atoms right after ionization. This leads the way to a better understanding and control of the motion of electrons in atoms and molecules.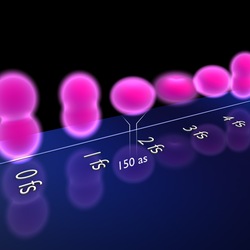
Stop-motion to see the electrons move. The ultra-high temporal resolution of 150 attoseconds enables the reconstruction of the evolution of the electron distribution of the krypton atoms right after ionization. Notice that this evolution occurs within a few femtoseconds only. Picture: Christian Hackenberger, LMU.
Attosecond research is nowadays at the forefront of technological and scientific progress. One attosecond is 10-18 seconds — a billionth of a billionth of a second: a truly tiny time interval, indeed. Many chemical and physical processes, ranging from simple electron dynamics in atoms to complex charge migration in biomolecules, happen at such a timescale. The steady development of attosecond technology, a natural consequence of ever-faster electronics and optics, is now opening the doors to the study of some of the fastest known processes happening in such incredibly short intervals of time.
"The basic principles behind attosecond technology are conceptually similar to stop-motion photography," explains Eleftherios Goulielmakis, who led the experiment at the Max-Planck-Institute for Quantum Optics in Munich (Germany). The image sensor (or film) constantly captures light reflected by a fast-moving object. A popular way amongst photographers to freeze the motion of such objects is to use a short exposure time to reduce the blur, and a flash to concentrate the light reflected by the object, within a short period of time. In the same way, achieving a high temporal resolution is arguably the most powerful way to experimentally study ultrafast processes.
Many groundbreaking ultrafast tools and techniques have been developed in recent years [1]. In particular, "attosecond laser pulses allow us to freeze motion," explains Adrian Wirth, who worked with Goulielmakis. "First, we eject one electron from a krypton atom by using a strong laser pulse. Then, after a specific time, we freeze the motion of the remaining electrons right after ionization, by using an attosecond laser pulse. In essence, our attosecond laser pulse thus acts just like a very sophisticated flash." In order to reconstruct the quantum mechanical wavefunction of the remaining electrons, an image is always taken of a large number of synchronized atoms. Finally, the dynamics of the process is reconstructed by combining a number of images taken at different stages of the wavefunction evolution. "The most intuitive way to think of the measurement process," continues Goulielmakis, "is by realizing that the distribution of the electron cloud around the ion influences how much of the attosecond pulse is absorbed. If the cloud is aligned like an antenna, it absorbs more radiation compared to when it is not. A detector behind the atoms measures the energy of the attosecond pulse at each snapshot and this allows us to reconstruct the motion."
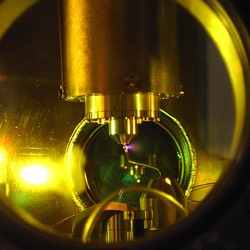
The eye of the camera. The look and working principle of an ultrafast apparatus differ radically from those of a conventional camera: krypton atoms are placed inside a vacuum chamber where they are ionized with a first laser pulse and subsequently probed by a second, ultra-short laser pulse. Picture: Eleftherios Goulielmakis, MPQ.
"This work is a perfect demonstration of the enormous potentialities of attosecond science, whose main goal is the real time observation and control of electron motion in atoms, molecules and solids," says Mauro Nisoli [2] from Politecnico of Milan (Italy). "As a consequence, we can now finally tackle several open theoretical questions experimentally. For example, it now becomes possible to study electron correlation effects or ultrafast multi-electron dynamics. On a more general level, a fundamental open question is whether the reaction pathways in a chemical reaction can be controlled and steered by controlling the electronic coherent motion on an attosecond timescale. If this turned out to be the case, we would soon see the new field of atto-chemistry emerge, expanding the current tools available through femto-chemistry." In fact, femto-chemistry has emerged as a consequence of new femtosecond technology about a decade ago. Today, femto-chemistry has matured into a standard way to influence chemical reactions with light and it is, for example, a powerful tool for laser-based isotope separation.
"Since the 1960s," Levine recalls, "we have seen rather regular improvements in our capabilities of dealing with ever shorter intervals of time. Back then, we were dealing with microseconds and microelectronics, today with attoseconds and advanced optics — and every three orders of magnitude somebody was awarded with the Nobel Prize." "Indeed," Nisoli agrees, "if attosecond technology proves to be useful in accessing the fascinating microcosmos microcosmos of ultra-fast processes, I am also convinced that it will have a tremendous impact on science and technology, and therefore receive its due credit." Apart from known ultrafast processes, both researchers are convinced that further, unexpected applications are bound to surface, thus expanding the impact of attosecond technology even further. "After all," Levine concludes, "I have always enjoyed working in this field because it is intellectually very challenging. And experience shows that, sooner or later, interesting science always leads to fascinating new applications."
[1] A. Niederberger, Towards Filming Chemical Reactions, Opt. Photon. Focus 2, 6 (2008) http://www.opfocus.org/index.php?topic=story&v=2&s=6.
[2] F. Remacle & R. D. Levine, An electronic time scale in chemistry, PNAS 103, 6793-6798 (2006).
[3] G. Sansone et al., Electron localization following attosecond molecular photoionization, Nature 465, 763-766 (2010).
Armand Niederberger
2010 © Optics & Photonics Focus
AN is currently working on his PhD on disordered ultracold quantum systems at ICFO - The Institute of Photonic Sciences in Barcelona (Spain).

Eleftherios Goulielmakis, Zhi-Heng Loh, Adrian Wirth, Robin Santra, Nina Rohringer, Vladislav S. Yakovlev, Sergey Zherebtsov, Thomas Pfeifer, Abdallah M. Azzeer, Matthias F. Kling, Stephen R. Leone & Ferenc Krausz, Real-time observation of valence electron motion, Nature (2010) 466, 739-743 (link).
The combination of images obtained using ultrashort laser pulses allows us to observe the evolution of the electrons of the krypton atoms right after ionization.