-
Viewpoint on 'IONS'
Viewpoint on 'Scientific Literacy'
- Proudly sponsored by
-

-
Updating the Size of the Proton: Small Difference, Big Consequence

The proton, one of the building blocks of matter, is considerably smaller than previously measured — latest studies suggest. This result may well challenge our current understanding of nature.
-
Subnanometer Perfection

According to French philosopher Voltaire (1694-1778) the perfect is the enemy of the good, thus not exactly desirable. As recent results show, however, where scientific research is concerned this may not always hold true.
-
The Disordered Quantum Prison
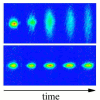
Imagine a prison without walls, an open field where prisoners cannot leave simply because the field is not smooth. This is the idea behind Anderson Localization and has recently been observed in ultracold atomic gases.
Volume 1 Story 5 - 10/7/2008
Physics needs experiments to validate its hypotheses. We develop concepts and models of particles, forces and natural constants. Yet, however elegant these may be, their accuracy can only be judged through experiments. Researchers at the National Institute of Standards and Technology (NIST) in Boulder, Colorado (USA), have built optical clocks that allow measurements with relative uncertainties of only 10-17. In a first experiment, they addressed a question of fundamental importance in trying to understand the history of our universe: they tested whether natural constants change with time.
In chemistry, as well as in everyday life, mass conservation is often taken as a fundamental hypothesis. Whether we determine the masses involved in chemical reactions or pour milk into our coffee, mass is conserved. Not surprisingly, observed mass conservation led to the assumption that exact mass conservation was a fundamental law of nature. When trying to develop a consistent picture of nature, however, we were forced to abandon this paradigm. High-precision experiments concluding that the speed of light is constant made it necessary to adopt the more advanced models and concepts of Einstein's theory of relativity. Amongst other profound consequences, these refined concepts imply that mass is not exactly conserved.
The example illustrates the importance of high-precision experiments and the importance of error bars: to advance science, we need to know the laws of nature as accurately as possible. As long as the error bars were larger than the effect itself, mass seemed to be conserved. Also, great achievements of chemistry and engineering have been possible believing mass is conserved. On a fundamental level, however, this assumption is simply wrong which has a profound impact on our understanding of nature.
Today, the most accurate measurements we can perform involve optical clocks. "If you want to measure something with extreme accuracy, you need to compare it to an extremely accurate reference," Till Rosenband from NIST explains. "In our setup, we drive an optical transition in ions, for which we have to set our laser to an extremely precise frequency. This gives us an extremely accurate reference that we can use to measure other quantities with unprecedented accuracy. Using aluminum and mercury ions, we have been able to measure up to the seventeenth decimal." This corresponds, for example, to measuring the distance to our closest sidereal neighbor, Alpha Centauri, to the precision where it matters if you perform the measurement from the floor or from a table.
"When measuring with such incredible accuracy, one of the most challenging tasks is to estimate and control the error bars," Rosenband points out. "For example, we had to cool our ions down to extremely low temperatures. Otherwise, atomic motion would have destroyed the accuracy of our experiments. And precision without accuracy is worthless!"
Having such an outstanding reference, the researchers have been able to improve the measurements of fundamental physical constants. The finestructure constant α, for example is of fundamental importance in quantum electrodynamics and it serves as an experimental parameter in the standard model of particle physics. In the present experiment they measured how much this constant varies over time and showed that α is constant to a degree that was never possible before. Such measurements are essential, for example, when trying to understand the history of our universe.
"Precision Measurements have always been a main line for Nobel Prizes," says Jun Ye, who carried out a similar experiment [1] using neutral atoms at the Joint Institute for Laboratory Astrophysics (JILA) in Boulder, Colorado (USA). These experiments are so complex that they always need the latest and most advanced techniques available. "For example", Ye points out, "the present experiments made use of several inventions recently awarded the Nobel Prize: trapping and cooling of ions, laser spectroscopy and just recently the frequency comb. Without these techniques, this kind of measurements would be impossible."
Apart from pushing the limits of science, high-precision measurements are also at the heart of many recent technological revolutions. The global positioning system (GPS), for example, makes use of precise measurements of distances to different satellites in order to deduce one's position. "The beauty of this kind of research," Rosenband adds, "is that we can do basic science and know that technological breakthroughs are just around the corner." And Ye concludes: "whenever you achieve doing something with extreme accuracy, there is a new tool coming that makes use of this accuracy. In this sense, it is fantastic knowing how, in our research, basic science and technological applications go hand in hand."
[1] A. D. Ludlow et al., Sr Lattice Clock at 1 x 10-16 Fractional Uncertainty by Remote Optical Evaluation with a Ca Clock, Science 319, 1805-1808 (2008).

Time to Test Physics
How can we be sure about anything in nature? Without experiments, all physics is merely speculation. Optical clocks now allow us to test science with unprecedented accuracy, refining our understanding of the universe.
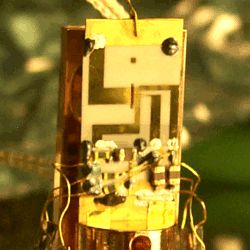
The heart of the optical clock. This part of the size of a fingernail contains the ion trap. Optical transitions of trapped aluminum and mercury ions serve as a reference, making measurements possible that are more accurate than ever before. Image courtesy of NIST.
In chemistry, as well as in everyday life, mass conservation is often taken as a fundamental hypothesis. Whether we determine the masses involved in chemical reactions or pour milk into our coffee, mass is conserved. Not surprisingly, observed mass conservation led to the assumption that exact mass conservation was a fundamental law of nature. When trying to develop a consistent picture of nature, however, we were forced to abandon this paradigm. High-precision experiments concluding that the speed of light is constant made it necessary to adopt the more advanced models and concepts of Einstein's theory of relativity. Amongst other profound consequences, these refined concepts imply that mass is not exactly conserved.
The example illustrates the importance of high-precision experiments and the importance of error bars: to advance science, we need to know the laws of nature as accurately as possible. As long as the error bars were larger than the effect itself, mass seemed to be conserved. Also, great achievements of chemistry and engineering have been possible believing mass is conserved. On a fundamental level, however, this assumption is simply wrong which has a profound impact on our understanding of nature.
Today, the most accurate measurements we can perform involve optical clocks. "If you want to measure something with extreme accuracy, you need to compare it to an extremely accurate reference," Till Rosenband from NIST explains. "In our setup, we drive an optical transition in ions, for which we have to set our laser to an extremely precise frequency. This gives us an extremely accurate reference that we can use to measure other quantities with unprecedented accuracy. Using aluminum and mercury ions, we have been able to measure up to the seventeenth decimal." This corresponds, for example, to measuring the distance to our closest sidereal neighbor, Alpha Centauri, to the precision where it matters if you perform the measurement from the floor or from a table.
"When measuring with such incredible accuracy, one of the most challenging tasks is to estimate and control the error bars," Rosenband points out. "For example, we had to cool our ions down to extremely low temperatures. Otherwise, atomic motion would have destroyed the accuracy of our experiments. And precision without accuracy is worthless!"
Having such an outstanding reference, the researchers have been able to improve the measurements of fundamental physical constants. The finestructure constant α, for example is of fundamental importance in quantum electrodynamics and it serves as an experimental parameter in the standard model of particle physics. In the present experiment they measured how much this constant varies over time and showed that α is constant to a degree that was never possible before. Such measurements are essential, for example, when trying to understand the history of our universe.
"Precision Measurements have always been a main line for Nobel Prizes," says Jun Ye, who carried out a similar experiment [1] using neutral atoms at the Joint Institute for Laboratory Astrophysics (JILA) in Boulder, Colorado (USA). These experiments are so complex that they always need the latest and most advanced techniques available. "For example", Ye points out, "the present experiments made use of several inventions recently awarded the Nobel Prize: trapping and cooling of ions, laser spectroscopy and just recently the frequency comb. Without these techniques, this kind of measurements would be impossible."
Apart from pushing the limits of science, high-precision measurements are also at the heart of many recent technological revolutions. The global positioning system (GPS), for example, makes use of precise measurements of distances to different satellites in order to deduce one's position. "The beauty of this kind of research," Rosenband adds, "is that we can do basic science and know that technological breakthroughs are just around the corner." And Ye concludes: "whenever you achieve doing something with extreme accuracy, there is a new tool coming that makes use of this accuracy. In this sense, it is fantastic knowing how, in our research, basic science and technological applications go hand in hand."
[1] A. D. Ludlow et al., Sr Lattice Clock at 1 x 10-16 Fractional Uncertainty by Remote Optical Evaluation with a Ca Clock, Science 319, 1805-1808 (2008).
Armand Niederberger
2008 © Optics & Photonics Focus
AN is currently working on his PhD on disordered ultracold quantum systems at ICFO - The Institute of Photonic Sciences in Barcelona (Spain).

T. Rosenband et al., Frequency Ratio of Al+ and Hg+ Single-Ion Optical Clocks; Metrology at the 17th Decimal Place, Science (2008) 319, 1808-1812 (link).